We have many kinds of pills available these days to treat all kinds of different disorders. Of course, the problem with pills is that they don’t work if you don’t take them. Even Worse, for some medicines, missing a dose can cause all kinds of undesirable withdrawl effects and set back a patient’s treatment.
Smart pills aim to fix this problem with a simple monitoring solution that can tell when a patient has taken their medication. They’re now publicly available and authorized for use, so let’s look at how they work.
They’re Putting Microchips In The Pills Now (Really!)
The development of the smart pill is very much a product of miniaturization. Modern electronics has advanced to the point where tiny sensors can be created in a size small enough to embed in a single pill.
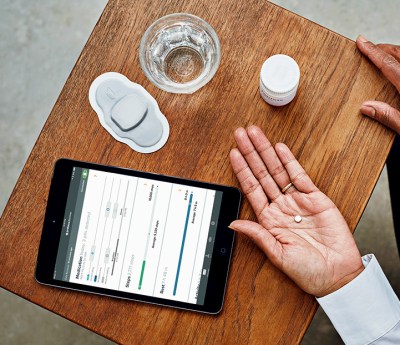
Perhaps the most well-known example is the medication known as Abilify MyCite, approved by the FDA in 2017. It’s a treatment that consists of an aripiprazole tablet with an included sensor that helps determine when a patient has taken the pill. The mechanism of action is ingenious. A tiny CMOS circuit is placed in the pill, along with a primitive battery cell. The battery cell is made up of magnesium and copper chloride. It is activated and releases its energy when the pill is in the presence of stomach acid, powering the CMOS circuit which sends a low-power modulated signal at a frequency of between 10 and 30 KHz, at a rate of approximately two packets per second. The signal is then picked up by a patch worn on the body, which sends a ping to a paired smartphone or tablet over standard Bluetooth. The components that make up the sensor are either harmlessly processed by the body or passed out as waste, with the sensor itself roughly the size of a grain of sand.
The system allows a smartphone to log when the patient takes a pill, updating the patient’s own records and sharing them with medical personnel. It also allows reminders to be sent if the patient forgets a dose, for example. By taking an automatic log, the system can help patients that may have issues remembering to take their medication. It can also alert doctors early in the case that a patient is missing their regular doses.
In the case of the Abilify MyCite pill, the medication involved is an ayptical antipsychotic drug by the technical name of aripiprazole. It’s primarily used as a treatment for conditions like schizophrenia, bipolar disorder, and obsessive-compulsive disorder. It’s a medication that can have negative withdrawl effects. The conditions it is intended to treat are also those that often come with issues around forgetting to take medications regularly. Thus, on paper, a system to track patients taking such a medication would appear to be a great tool to avoid negative outcomes.
Despite this, the Abilify MyCite monitoring system has raised significant concerns around patient privacy and safety. To ease this, thus far, patients using the system must sign consent forms allowing doctors or family members to monitor the system. The smartphone app will also allow the patient to block the sharing of this data at will.
However, there are concerns that such technology will enable more invasive activity from the healthcare system. Insurers could theoretically require such systems for patients requesting certain medications, and penalize those that don’t comply with their treatment scheme. Similarly, patients on release from psychiatric care could be threatened with involuntary admission for not adhering to their prescribed dose schedule. This comes with the risk that a malfunctioning pill or sensor patch could unfairly punish even “compliant” patients. Even worse, conditions like schizophrenia often come with symptoms of paranoia, particularly around surveillance and technology. Literally putting microchips into the pills to treat the condition won’t help in that regard.
There are also questions around whether or not the system will actually help patients to take their medications more regularly. Typically, medical professionals talk in terms of “patient compliance” with treatment. However, the sensors aren’t a sure-fire solution to this. Some have noted that patients could induce vomiting after taking the medicine in an attempt to fool the system. Alternatively, it’s plausible that dropping a pill into something approximating stomach acid may also trigger the chip inside to send a positive signal. Notably, the FDA notes from 2017 admit that the system hasn’t been proven to “improve patient compliance with their treatment regimen.”
Overall, smart pills are an amazing piece of medical engineering. Being able to sense when a pill has been ingested in a non-invasive manner is an impressive technological feat. As with so many new developments, though, there are heavy ethical concerns to contend with. Expect smart pills and their usage to become a shifting battleground between doctors, patients, and insurance companies in the years to come.
Smart Pills Can Tell Your Doctor That You’ve Taken Them
Source: Manila Flash Report
0 Comments